The Struggles of Fast-Charging Lithium-Ion Batteries
One of the struggles of electric cars is the charging time. Even though I'm a battery-geek, I get it. Why would you want to wait hours on charging the battery if you can fuel the car in 5 minutes? Is it really that hard to charge a battery faster? I decided to look into this problem when I was an exchange student at NTNU... I was quite surprised with my research. Maybe a 15 minute charging time in the near future isn't that unrealistic after all?
Fast-charging lithium-ion batteries would be well-welcomed for electric vehicles users and manufacturers. A fast-charging battery might even give the driver a "fuelling"-experience in terms of convenience. The U.S Advanced Battery Consortium (USABC) has a goal of batteries in electric vehicles to be capable of charging 80 % within 15 minutes by 2023 [1]. However, the conventional graphite electrode has limitations regarding fast-charging due to slow intercalation kinetics [2]. Many different approaches have been taken to solve this issue, including coatings and surface engineering[2][3]. However, this post focuses on more direct modifications of the graphite in the form of structural modifications and graphene sheets.
Intercalation of Lithium-Ions into Graphite
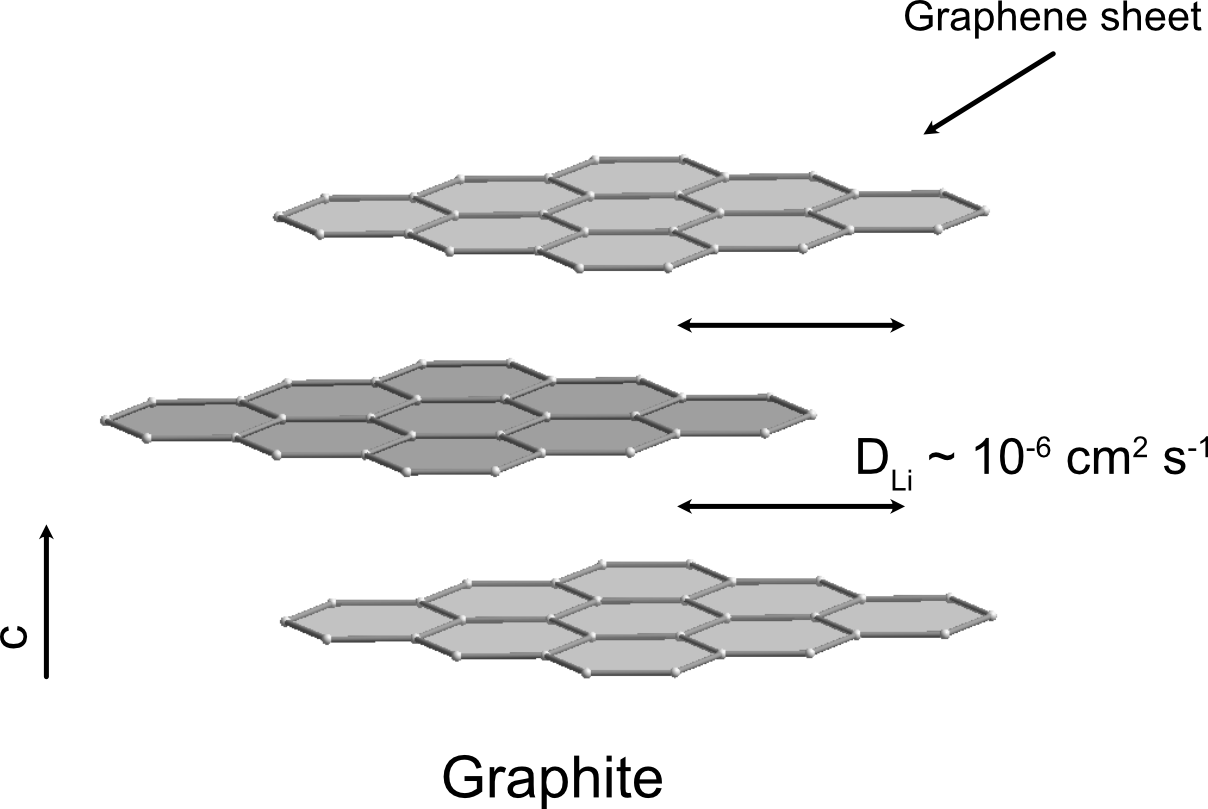
When a secondary lithium-ion battery is being charged, the lithium is ideally intercalated via the (0002) planes of the graphite, as seen in Figure 1. The lithium diffusivity in this direction is relatively fast, DLi =10-6 cm2 s-1 [4]. However, graphite is an anisotropic material, which means the material's properties depend on the direction of the crystal structure [5]. Graphite electrodes typically consist of grains with different orientations, leading to some lithium-ions being intercalated via the less optimal c-direction. The intercalation rates in this direction can be 4-6 orders of magnitude lower compared to intercalation via the (0002) plane. This lack of perfect orientation means that, in practice, the lithium diffusivity does not reach its highest. Researchers believe that the intercalation of lithium into graphite in the c-direction is essentially impossible and only takes place due to defects or along the grain boundaries [4].
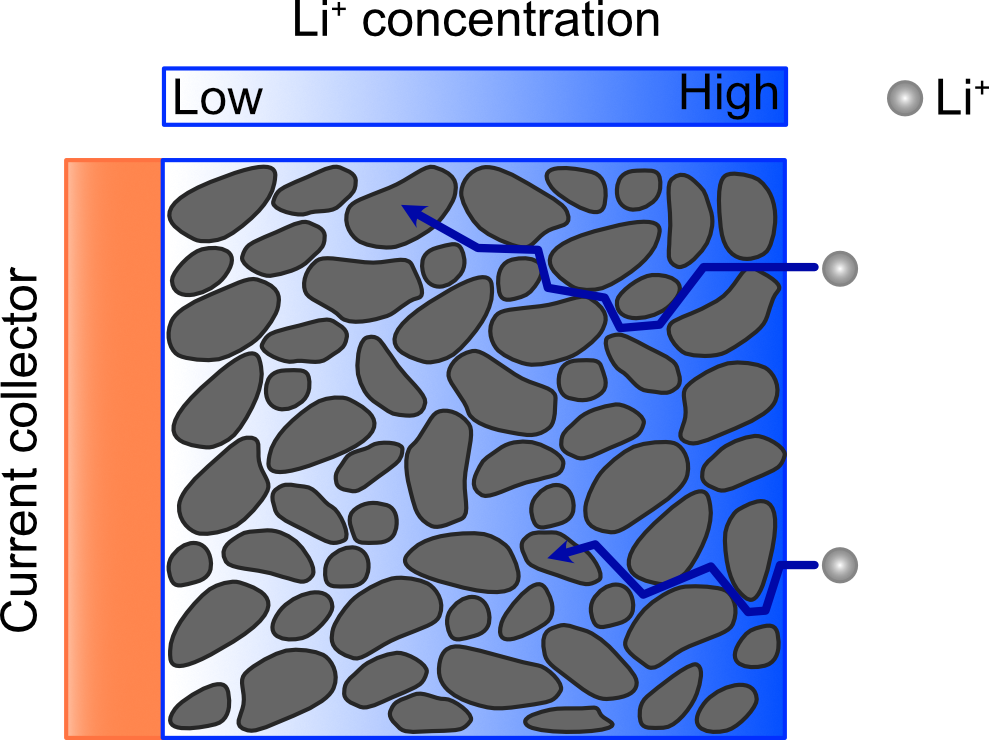
The thickness of the electrodes causes another reason for slow charging rates for Li-ion batteries. The thick electrodes cause transport limitations as they form tortuous diffusion paths for the lithium ions. This leads to a lithium concentration gradient across the electrode as shown in Figure 2 [1]. As illustrated, regions of the negative electrode near the current collector are not fully utilized [6]. This concentration polarization can cause lithium plating to become electrochemically favorable, forming irreversible lithium dendrites on the electrode surface [1].
Laser-Patterned Pore Channels
One can take many different approaches to overcome the challenges of fast charging a battery. In a paper from 2020 by Kuan-Hung et al. [1] used laser-patterning to create arrays of pore channels in the negative graphite electrode. They name these highly ordered laser-patterned electrodes for "HOLE". The pores serve as diffusion channels of the lithium and reduce the concentration gradient across the electrode upon charging. When cycling the HOLE cell, the research group found a capacity retention of 97.2 % after 100 cycles of charging at a 4C-rate. The unpatterned control electrode had a capacity retention of just 69 % after being cycled under the same conditions. When cycling the HOLE cell 100 cycles at a 6C charging rate, the capacity retention was 93.4 %, while the control electrode had a capacity retention of 59 %. These HOLE-electrodes have an advantage over many alternative approaches, as they can easily be incorporated in the current manufacturing of graphite electrodes by adding an extra step to introduce the laser-patterning [1].
Graphene Sheets
Another approach to reach fast-charging lithium-ion batteries was taken by Mu et al. [7]. They published a paper in 2020 where they grew vertical graphene sheets, VGSs, on the surface of natural graphite. These VGSs increase the lithium-ion transport rate both by their intrinsic high electrical conductivity and by creating diffusion paths with a lower degree of tortuosity. Mu et al. used cells with LiFePO4 as positive electrode material. The mass percentage of the VGSs was determined by measuring the mass increase after growing the VGSs on the pristine graphite. It was found to be 31.2 %. The researchers cycled both VGSs/graphite cells and pristine graphite cells at a 5C-rate with a charging time of just 3 minutes. They found the cells with pristine graphite delivered a capacity of just 9.4 mAh g-1 (at the final cycling) with a capacity loss of 0.0773 % per cycle during 1000 cycles. The cells with VGSs/graphite delivered capacity of 105.4 mAg g-1 (at the final cycling) with a capacity loss of 0.0093 % per cycle during 3000 cycles [7].
Both of these approaches show great potential as modifications of current graphite electrodes. Perhaps the goal by USABC is not as optimistic and unrealistic as one might think.
[1]: Chen, K. H., Namkoong, M. J., Goel, V., Yang, C., Kazemiabnavi, S., Mortuza, S. M., ... & Dasgupta, N. P. (2020). Efficient fast-charging of lithium-ion batteries enabled by laser-patterned three-dimensional graphite anode architectures. Journal of Power Sources, 471, 228475.
[2]: Kim, N., Chae, S., Ma, J., Ko, M., & Cho, J. (2017). Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nature communications, 8(1), 1-10.
[3]: Kim, D. S., Chung, D. J., Bae, J., Jeong, G., & Kim, H. (2017). Surface engineering of graphite anode material with black TiO2-x for fast chargeable lithium ion battery. Electrochimica Acta, 258, 336-342.
[4]: Mukhopadhyay, A., Guo, F., Tokranov, A., Xiao, X., Hurt, R. H., & Sheldon, B. W. (2013). Engineering of Graphene Layer Orientation to Attain High Rate Capability and Anisotropic Properties in Li‐Ion Battery Electrodes. Advanced Functional Materials, 23(19), 2397-2404.
[5]: Marsh, H., & Reinoso, F. R. (2006). Activated Carbon. In Activated Carbon. Elsevier.
[6]: Chen, K. H., Goel, V., Namkoong, M. J., Wied, M., Müller, S., Wood, V., ... & Dasgupta, N. P. (2021). Enabling 6C Fast Charging of Li‐Ion Batteries with Graphite/Hard Carbon Hybrid Anodes. Advanced Energy Materials, 11(5), 2003336.
[7]: Mu, Y., Han, M., Li, J., Liang, J., & Yu, J. (2021). Growing vertical graphene sheets on natural graphite for fast charging lithium-ion batteries. Carbon, 173, 477-484.